

Diamonds and the Engineer
Apart from their intrinsic value as precious stones, diamonds are of almost inestimable worth to the engineer, for they are indestructible and will cut their way through the toughest steel and the hardest stone or rock

THE PREMIER DIAMOND MINE was opened about twenty-
THE story of the diamond is full of romance; love and murder, pageantry and robbery, intrigue, superstition and sentiment all play their parts in it. This aspect of what, after all, is only crystallized carbon, can well be left alone here, but no account of modern engineering would be complete without some reference to the part the diamond plays in it. Most people will have seen the glazier’s diamond and may think that this is the only practical application of the precious stone, an assumption far from the truth.
In the tool-
The object of the machine, which is a comparatively recent invention, is to ascertain the degree of hardness of metal parts which are subject to wear and stress: for instance, the teeth of certain gear wheels. Hardness is a property which is not detectable by the other tests to which metals are subjected — such as those for ascertaining the tensile strength, the elasticity, resistance to fatigue and so forth. A special test had, therefore, to be devised for it.
This test consists of making a small impression on the material by an indenting point and then measuring the diameter of the impression, which will be larger in a soft than in a hard material. The load used to produce the impression being known, a simple calculation will give a figure indicating the degree of hardness of the specimen. All tests for hardness are only relative, that is, the result is not given in any system of units, such as pounds per square inch, but in an arbitrary figure, which, however, enables the specimen to be rejected or accepted according to its degree of correspondence with a standard table.
The indenting point in the hardness testing machine consists of a diamond accurately cut and polished to the form of a square-
This impression is then examined through a microscope fitted with a micrometer measuring device and the width across the corners is read off to a degree of precision as fine as one-
On looking through the microscope the operator sees a pair of knife edges, one on either side of the image of the impression. He adjusts the micrometer until-
The load is applied by a system of weights and levers and may be anything from 1 kilogramme (2.2 lb.) to 120 kilogrammes (264 lb.), according to the particular class of material being tested. It is not desirable to use too heavy a load or the impression may be so large as to be unsightly and, as the test is often applied to a finished piece of work, it is generally left as it is and not ground out. The hardness testers using diamonds are not invariably of the type just described. There are several different types and, moreover, in some machines a hard steel ball is used as the indenting point. The principle of the hardness tester is, however, the same in all the machines.
In engineering shops machines may be noticed in which circular parts, shafts and so forth, are being finished with a beautifully polished surface and to a high degree of accuracy. These are grinding machines in which artificial grindstones, rotating at enormous velocity, are pressed against the work which itself rotates so that a truly circular form results. But the stone may wear unevenly; at intervals, therefore, a diamond mounted on a hinged bracket is traversed across its face to cut it true again.
In some machines of this kind — the so-
In another part of the shop may be seen some machines resembling lathes. They are a form of lathe, but instead of the usual saddle carrying a steel or tungsten carbide tool, there is a different structure finished with a diamond which takes a light cut off already machined work, giving it a mirror-
This type of machine is often used to bore out holes which have to be finished with great precision. A diamond is used as a cutting tool because of its superlative hardness; for it does not become blunt, as does a steel tool, which needs frequent regrinding. It is the quality of hardness that makes the diamond valuable in the hardness testing machine and for “truing” grinding wheels.

CRUSHING PLANT at the Premier Mine near Pretoria, Transvaal. Ground from the mine is disintegrated and fed into washing pans filled with muddy water. The concentrates are then passed over shaking tables such as those illustrated at the bottom of this page.
The diamond is the hardest substance known, twice as hard as topaz or corundum. Its high refractive index (ratio of the sines of the angles of incidence and refraction) gives it brilliance, its high dispersion of light that wonderful fire. It is transparent to light and to X-
Diamonds crystallize in the Cubic System, generally in the form of an eight-
Certain varieties of diamond are dissimilar in appearance, but chemically identical. Bort, or boart, is a rough, greyish aggregation, rounded in form, sometimes with radiating structure. It is found in the interior of Brazil and, though useless for jewellery and apt to cleave, is important as an abrasive. Carbonado, also from Brazil, is a black, micro-
Artificial Diamonds
As diamonds have been found in meteorites, both of the stony and of the metallic types, in Russia, Hungary, Chile, Arizona and Western Australia, Sir William Crookes (1832-
Diamantina was later rivalled by Bahia, where, at the beginning of the century, several thousand men were employed in the workings; it is the centre for carbonado. In British Guiana men collect diamonds from the streams. The occurrence of diamonds in Borneo has long been known, and they were found in South Australia in 1851. They have been reported from the Urals, Lapland, and parts of Siberia and of the United States.
In 1867 Dr. W. G. Atherstone recognized as a diamond a pebble picked up by a child on the banks of the Orange River in South Africa; the diamond was sold for £500 and exhibited at the Paris Exhibition. Two years later a stone of 83 carats was found near the same river. It was named the Star of South Africa and bought by the Earl of Dudley for £25,000.
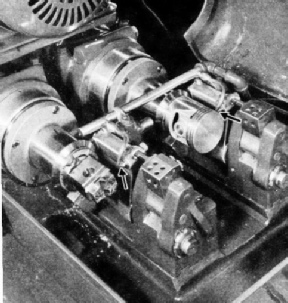
FINISH TURNING of aluminium pistons by a diamond tool. The machine deals with two pistons at a time, but only one,
on the right, is in position. The chuck for holding the piston is seen on the left. The diamonds are fixed in steel holders, and their positions are indicated by the points of the black arrows.
That discovery led to a “rush”, and before long there had sprung up a mining camp of 10,000 men on the River Diggings, along the Vaal and Orange Rivers. Here the search was carried on in the river gravels by the time-
Towards the end of 1870 diamonds were found at other localities, at Jagersfontein in the Orange Free State, and at Du Toit’s Pan, both far from the river. This led to a second rush, and another mining camp sprang up near Du Toit’s Pan. Diamonds were also found near by, at Bult Fontein and in two places near Vooruitszicht. This was the beginning of the mines that became famous under the name De Beers and Kimberley, all within a circle of five miles diameter.
Here conditions were different, for the diggings were dry. The rich ground was always in a circular patch, quite small, often in those round “pans” so well known in South Africa. Beneath the red soil diamonds were found in the “yellow ground”, and, lower still, in a hard, bluish-
South African “Pipes”
These round patches became known as “pipes”, and such pipes are found elsewhere in South Africa, though not always containing diamonds. Their origin has been as much debated as that of the diamonds themselves. It is clearly not alluvial, and is thought by some to be volcanic, but there are difficulties in that theory, too.
The industry developed so rapidly that by the early years of this century the output of the Orange Free State was 320,000 carats, valued at £938,000. By then South Africa was producing ninety per cent of the diamonds of the world.
In 1908 diamonds were discovered in South West Africa. In the centre of the continent large quantities were won in the Belgian Congo and in Angola. Diamond deposits have been reported also from Tanganyika and Southern Rhodesia.
In spite of the rivalry of the pipes, the alluvial diggings remained alive, chiefly in the southwest of the Transvaal. In diggings near the Premier Mine was found the Jonker diamond, weighing 726 carats raw. (An English carat is 906 equal to 3.17 grains; 151½ carats equal one ounce.) The Jonker diamond was sold for £63,000, but is now valued, in the United States, at £250,000. In 1926 there was a rush to the Lichtenburg district, in the Transvaal, and in Namaqualand, South West Africa, great quantities were produced.
The working of gravels in India was studied by a French jeweller named Tavernier in 1645. He found about 60,000 low-
In the South African river diggings the methods first used were scarcely more advanced. In the dry diggings each claim was an independent pit, 31 feet square, sunk through the yellow into the blue ground. The earth was hoisted out with a windlass, and narrow roadways were left to provide access between the claims. Gradually the excavation ate back into the roadways, which became unsafe and collapsed, so that the whole place developed into one colossal pit.
The only way the diggers could excavate their ground was by hoisting from a windlass on the rim. There was thus formed an enormous abyss, with a triple row of windlasses round the edge, each connected by a wire rope to a bucket hoist down in the depths. The ropes from the highest tier of windlasses reached out into the middle, and those from the lowest worked the sides. The windlasses were worked by horses at first, but steam was introduced in 1873. By 1874 there were 11,000 men at work at Kimberley.

THE GREAT HARDNESS OF THE DIAMOND is useful to the engineer in many ways. The disk on the left (1) is a die used for drawing fine wire. The wire is pulled through a small hole drilled in a diamond which is mounted in the centre of the die. The illustration on the right (3) shows a turning tool of steel, whose cutting edge, however, consists of a diamond with a sharp edge. In the centre (2) is the nozzle of an oil burner for a boiler. Oil is forced at high pressure through a small hole into the furnace. The hole is drilled in a diamond, no other material being able to withstand the erosion of the oil stream so effectively. The holder is of steel.
The first claims had been pegged on July 16, 1871. Four years later the crater had reached a depth of 100 feet, and then difficulties began. Water accumulated in the bottom. The sides exposed to the weather began to disintegrate and fall in, and many claims were rendered unworkable. By 1882 more than half the claims were buried, and by the following year the continuation of the work had become almost impossible. The other mines had a similar experience and by 1889 Kimberley, Du Toit’s Pan, De Beers and Bult Fontein gave up open cast work and started mining in the true sense.
By that year Cecil Rhodes and Alfred Beit had completed their control of the De Beers and Kimberley mines, and soon afterwards consolidated the whole group into one organization, De Beers Company. Thus the work was centralized.
The men and network of wire cables were withdrawn from the crater, which then bore the appearance of a deserted chasm. Shafts were sunk outside the pipe, from which roads were driven into the pipe at different levels, separated by 40 feet intervals. In the blue ground itself, parallel tunnels were cut at 120 feet intervals to the opposite side of the pipe, and at right angles to these another set of tunnels at 36-
All the mines had similar experiences. De Beers, a mile to the east of Kimberley, was also driven to adopt underground workings and reached a depth of 2,400 feet. Work was discontinued here in 1908. Bult Fontein, about two and a quarter miles south of De Beers, worked by open cast down to 700 feet, and then carried by true mining down to 1,600 feet.
Breaking Up the Blue Ground
Bult Fontein Mine, covering about thirty-
At Jagersfontein, in the Orange Free State, work by the open cast system was easier because of the hard basalt walls of the crater, which withstood the weather well, but in 1910 a deep shaft was sunk on the Kimberley system and a second shaft shortly afterwards.
Open work was continued on a grand scale at the Premier Mine, twenty-
The primitive method of the dry diggers was to break up the hard blue ground with mallets to extract the diamonds. This was laborious, expensive and inefficient. The first step forward occurred when it was found that the blue ground weathered readily. It was then carted out into open country and spread on the ground, exposed to the sun, wind and rain. This disintegrated it effectively in a few months. The broken-
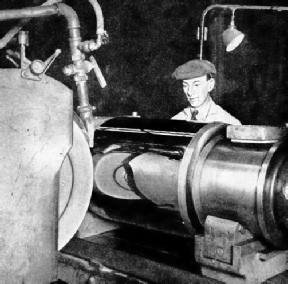
FINISHING A STEEL MILL ROLL in a grinding machine. The grinding wheel on the left rotates at a high speed. The roll being ground also rotates and the wheel and the roll have relative axial traverse so that the whole surface is covered. The remarkably high degree of finish attained in the machine is seen by the reflection of the wheel and its guard in the mirror-
As the industry expanded, fortunes were made. The value of claims rose from a hundred or so to many thousands of pounds. The richer bought out the poorer, and consolidation increased efficiency. A Mining Board was formed to protect the interests of the industry. Bucket elevators were introduced to carry away the tailings and the scanty water supply was properly organized.
The winding and washing plants were modernized. The ground was hoisted six truckloads at a time, that is 96 cubic feet, and hauled from the pit’s mouth to the “distributing floors”, where it was exposed to the weather. The disintegrated ground was then fed through perforated cylinders into washing pans, after any lumps which had resisted weathering had been specially broken up. These pans are shallow cylindrical troughs filled with muddy water. The heavier minerals are washed to the sides by revolving toothed arms, the tailings escaping by the centre. The concentrates are then passed over shaking tables, to concentrate them still further. After that they are washed on a sloping table of corrugated iron covered with grease, to which it has been found that diamonds adhere, the dirt being washed away to the tailings. At the Premier Mines the Elmore process of mineral separation by flotation in oil was introduced.
The Driller’s Crown
Diamonds are essentially concentrated value, more so even than gold, and an enormous amount of dirt must be mined and treated to recover but a small quantity of the precious stones. The blue ground not only carries isolated stones often of immense value, but is impregnated also with microscopic diamonds. Yet the average yield was only from 0.2 to 0.6 carats per load of 1,600 lb., or an average of about only one and a half grains per ton.
Another highly valued commodity — gold — has been largely responsible for increasing the demand for diamonds. Prospecting for new goldfields generally involves much rock drilling, for which bort is necessary for the crowns of the rotary drills. To reduce expenses, specially hardened steels and chilled shot have been tried, but these can replace bort only partly, for nothing but diamond in some form will resist the tremendous wear and tear of drilling through hard rocks. The driller’s crown is a ring of steel, into the face of which are set diamonds in such a manner that small angles project over the surface of the steel. These projections rub against the rock when the crown is rotated, and so wear it away.
The embedding of the stones in the crown calls for considerable skill. For this work bort is used, as the cost of jeweller’s stones would be prohibitive. As it is, a crown may contain more than £400 worth of stones. There is a form of crown in which a dozen or more small stones are used instead of four or five big ones, and the outlay is considerably reduced. Bort, in the same way as clear diamonds, is brittle, and so may be broken in irregular, lumpy ground, or torn out of the steel by the tug of tough clays, or the whole crown may be lost bodily at the bottom of a borehole. If therefore there had been any substitute for diamond, drillers would have found it long since.
A somewhat similar use of the diamond is for that of sawing stone of all kinds. The arrangement used for this task is similar to that of a rotary drill in so far as the stones are set in a metal disk. The saw resembles the ordinary circular saw for cutting wood or metal but round its periphery are set numerous steel holders. These are dovetailed into the disk and securely fastened with their surfaces flush with the periphery, the diamonds alone standing clear of it.
The disk is thin but, even so, it would not function as a saw if the protruding diamonds were all set in line round it. They are therefore “staggered” in groups of three, so that the cutting effect extends right across the disk. Thus one diamond is at the left-
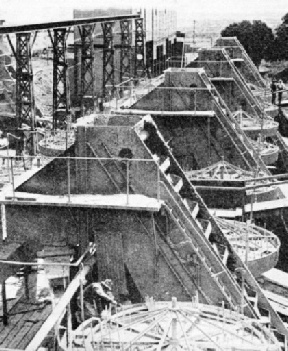
WASHING PLANT at the Premier Mine, near Pretoria, Transvaal. An enormous amount of dirt must be mined and treated to recover but a small quantity of the precious stones. The average yield is little more than 0.6 carats in every 1,600 lb.—about grains per ton.
The diamond saw revolves at a high speed and cutting with it is therefore rapid. When Portland stone is being cut the diamonds go through it more rapidly than through such a material as Sicilian marble. Slate comes about half way between the two, an example with this material being a speed of 6 in. a minute through a block 12 in. thick with a saw of 40 in. diameter and needing 12 horse-
The demand for diamonds in the motoring industry is for the purpose of the manufacture of fine screens for oil filters. These require to combine the maximum of strength with the minimum of thickness, so that a screen of exceptionally fine mesh may be made, which will stand the strains put upon it in the constant cleaning, and also be proof against corrosion. Identical demands are made by many manufacturers of foods, and the demand is growing constantly.
Until recently the only material that would fulfil these requirements was platinum, of which the cost is excessive, but the development of modern stainless steel has provided an adequate substitute at a cheap price. Nowadays ideal fine mesh screens can be made of a variety of steel which is immune from corrosion, yet has all the strength of steel. The incredibly fine wire necessary for these meshes of microscopic size can be drawn only through dies of diamond, for nothing else can give the constancy of diameter in such fine dimensions.
The rod of metal, is first rolled through reduced diameters under steel rolls, and then the process is repeated on a much smaller scale, constantly reducing. For diameters down to one eighth of an inch, tungsten carbide has replaced diamonds for the dies, but below one sixteenth,diamond is irreplaceable. The wire may be pulled through four or five such dies, each slightly smaller than the last, at the surprising rate of nearly 3,000 feet a minute. The diamond is so hard that it resists the wear, and the wire is produced with constant diameter. Few materials but platinum or steel would stand the strain.
The stones used for making these dies are diamonds rejected by jewellers because of some, blemish, but the diamonds must be free from flaws. Neither bort nor carbonado is suitable.
“Diamond Cut Diamond”
The hole through which the wire is drawn must be circular, and to drill such a hole one four-
Most of the stones for this purpose come from France, but the dies themselves are made by several firms in Great Britain, though there are few that can make dies of less grade than 0.002 in. The diamond, here called the “nib”. is held firmly in a “hoop” of steel, to counteract the bursting strain to which it is exposed. One facet is polished, to serve as a window, through which the process of the drilling can be watched. The drilling is done with the finest diamond dust, held in pure olive oil, at the end of copper wire, in which the dust is embedded by the pressure. This is an application of the old principle, “diamond cut diamond”, on which is based the art of the lapidary. A die of medium grade may cost about 30s., but one of a finer grade will cost about £7. In the finest diameters the price rises again because of greater labour cost.
Below a diameter of 0.001 in. the diameter of the hole is no longer truly circular, but in such minute dimensions, which convey little to the layman, theory dominates practice. The diameter of such a wire, for instance, is not measured directly, but indirectly, by calculation based either upon the length, strength and weight of the wire, or upon its electrical resistance.
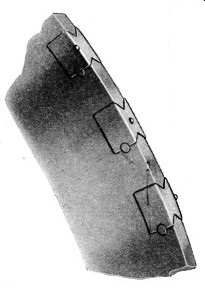
EDGE OF A DIAMOND CIRCULAR SAW for cutting stone, slate and the like. The diamond holders are dovetailed into the rim of the saw and the diamonds themselves are mounted in different positions in recurring angles all round the circumference. The bottom diamond is seen on one edge of the saw rim, the one above that is at the centre and the top one at the other edge. The next diamond would be at the centre and so on.
The passage of wire through a diamond is not the only instance providing resistance to wear due to movement by means of this remarkable substance. Strange as it may seem, a jet of oil under pressure passing through an orifice will quickly wear away such a material as steel. In oil-
Other purposes for which diamonds are used in industry and the arts, apart from those already mentioned, are for cutting glass and porcelain, for fine engraving, for dentists drills, for turning tools for electric light carbons, for highly accurate turning of astronomical and other instruments of precision, and for bearings of watches and electrical meters.
The craft of the lapidary has been known for thousands of years, but it was not until 1475 that the art of cutting the hardest of the gems was invented by one Louis de Berquem, of Bruges, who worked in Amsterdam and Antwerp.
Stone of 3,025¾ Carats
The rough stone must pass through an elaborate process before assuming its full beauty. First, to handle the stone, the worker sets it firmly in some alloy that fuses at a low temperature in a hollow at the end of an iron handle called the “dop”. Then lumps and irregularities are ground off by rubbing two diamonds against each other. This is called brutage.
The next process is cleaving, to eliminate flaws and yet produce the biggest stone possible. This is a delicate business, calling for the greatest skill, care and judgment. Advantage is taken of the tendency to split along defined planes. The facets are produced by grinding two stones together.
Diamond cutting forsook London long ago for Amsterdam and Antwerp, but it is well developed in New York, where experts learnt to saw stones.
There is a highly specialized technique. A brilliant is a diamond so cut that it has a flat top, the “table”, and a smaller flat base, the “cullet”, with fifty-
The most famous instance of diamond cutting was that of the Cullinan. This extraordinary stone was discovered in 1905, in the “yellow ground” in the Premier Mine, and still challenges the world. Three times the size of any diamond hitherto recorded, the raw stone weighed 3,025¾ carats. It was perfectly clear and water-
The diamond was quite flat on one side, a fact which suggests a cleavage plane; in other words, that this giant was but part of a once still greater stone. It was bought by the Transvaal Government in 1907 and presented to King Edward VII.
The cutting of this stone was done in Amsterdam by the old-
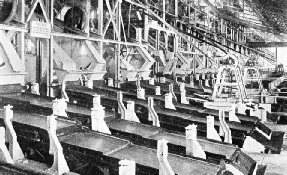
SOME OF THE PULSATOR TABLES of a mine at Kimberley, South Africa. Concentrates from the washing pans are passed over shaking tables, or pulsators, and again washed, on inclined tables covered with grease, to which the diamonds adhere.
You can read more on
“Romance of African Copper” and
on this website.